The healthcare regulatory bodies like AMA and Medicare keep on updating the billing and coding guidelines and regulations.
The healthcare providers must stay updated with the latest trends and changes related to billing and coding.
In a recent development, American Medical Association (AMA) just updated the CPT code sets for vaccines of 2024-2025 influenza season.
The AMA releases the updated code for respiratory illness including COVID-19 and influenza.
According to CDC, vaccination protects against severe outcomes of COVID-19 and flu, including hospitalization and death. In 2023, more than 916,300 people were hospitalized due to COVID-19 and more than 75,500 people died from COVID-19. During the 2023-2024 flu season, more than 44,900 people are estimated to have died from flu complications.
Trivalent Vaccine for the 2024-2025 Season
The public health community is once again marshalling its defenses against influenza by formulating updated vaccines for the 2024-2025 flu season. This year’s trivalent vaccine will contain antigens to protect against three targeted viral strains: a version of H1N1, H3N2, and a component from the B/Victoria lineage.
This combination represents the experts’ best efforts to anticipate the strains most likely to spread through communities in the coming months. The vaccine spurs the body to generate protective antibodies against these three targets. Though the circulating flu strains often mutate and evade the vaccine’s defenses, immunization still reduces severe outcomes.
Flu Vaccine Code Changes
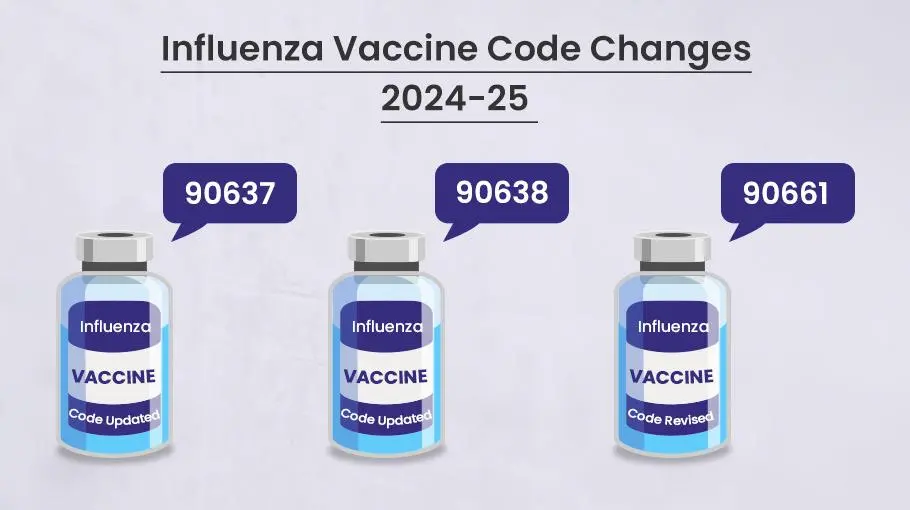
Medicare has yet to publish the payment rates and effective dates for the 2024-2025 flu season. However, there are a couple of new flu vaccine codes that will take effect on July 1st, 2024 worth taking note of.
The first is code 90637 for a quadrivalent influenza mRNA vaccine with a 30 microgram per 0.5 mL dose for intramuscular injection. The second is code 90638 for the same quadrivalent vaccine but with a 60 microgram per 0.5 mL dosage. Both of these new vaccines are still pending approval by the FDA.
There is also a revised code to make note of. Code 90661 is for a trivalent influenza vaccine derived from cell cultures that is preservative and antibiotic free with a 0.5 mL dose for intramuscular use. The descriptor for this code will change effective January 1st, 2025.
| New Code | Details |
| 90637 | Influenza virus vaccine, quadrivalent (qIRV), mRNA; 30 mcg/0.5 mL dosage, for intramuscular use |
| 90638 | 60 mcg/0.5 mL dosage, for intramuscular use |
| 90661 [revised] | Influenza virus vaccine, trivalent (ccIIV3), derived from cell cultures, subunit, preservative and antibiotic free, 0.5 mL dosage, for intramuscular use |
The schedule of Medicare coverage for the looming flu season is still uncertain in 2024. However, two new vaccination codes have already made their debut on July 1st. The first, code 90637, is for a quadrivalent influenza vaccine with an mRNA dosage of 30 micrograms per half milliliter, meant for intramuscular injection. The second, code 90638, specifies the same quadrivalent vaccine but with double the dosage at 60 micrograms. Approval from the Food and Drug Administration for these vaccines is still pending.
Other Vaccine Code Changes in 2024
The American Medical Association has approved some new vaccine codes for the 2025 edition of the Current Procedural Terminology manual. This reference work provides medical codes that physicians and other providers use for billing and record-keeping.
One of the newly accepted codes is 90684, for the 21-valent pneumococcal conjugate vaccine given by intramuscular injection. The Food and Drug Administration approved this vaccine on June 20, 2024, and the new CPT code is effective as of June 17, 2024. This shot protects against 21 strains of the bacterium that causes pneumococcal disease.
There is also a new code, 90624, for a combination meningococcal vaccine given intramuscularly. This pentavalent vaccine includes components against meningitis types A, C, W, and Y plus recombinant proteins against type B disease. It is pending approval from the FDA and will take effect on October 1, 2024 assuming it gets the green light. This should provide broad protection against all major forms of meningitis in one shot.
| Other New Codes | Details |
| 90684 | The 21-valent pneumococcal conjugate vaccine given by intramuscular injection |
| 90624 | The combination meningococcal vaccine containing components against meningitis types A, C, W, and Y plus recombinant proteins fighting type B disease |
FDA Approves RSV Vaccine
The FDA has given the green light to Moderna’s respiratory syncytial virus vaccine, approving it for use on May 31st. This new mRNA vaccine, delivered via lipid nanoparticles into the arm muscle, will be assigned the CPT code 90683 when it is published in the 2025 edition of CPT.
Meanwhile, RSV-positive test results and hospitalizations have remained low nationwide, as have vaccination rates, according to the Centers for Disease Control and Prevention.
The CDC continues monitoring influenza-like illnesses as we move into summer, when most respiratory viruses take a break. With flu season in the rearview mirror, it seems we’ve dodged a viral bullet this year. But it’s never too early to start thinking about getting next season’s flu shot, especially for those at high risk of complications.
Guidelines for Medical Facilities and Providers
🔵 Doctors could recommend both the COVID-19 and influenza vaccinations during the same visit if the patient needs both.
🔵 As long as the physician billed for both vaccinations together when a patient received them at the same appointment, there wouldn’t be any duplicate charges.
🔵 The medical practices simply have to remember to use the correct, up-to-date codes for the vaccinations to prevent claims from being denied by insurance companies. They also need to accurately document the names of the influenza strains along with the corresponding codes.
🔵 The billing software systems need to be updated to properly translate the diseases and bill them correctly.
🔵 The official website of the American Medical Association, as well as various medical billing blogs, should be checked regularly for the latest updates, changes, and other important details regarding the new vaccine codes.





